Six Easy Pieces
Essentials of Physics Explained by Its Most Brilliant Teacher (Richard P. Feynman)
Bold = new section
Italic = particularly interesting term or phrase
Underline = important vocabulary
Links are included throughout. I have purposefully varied the sources and the format (text, image, audio, video) so that my own notes are not only expanded on, but enriched! The links won’t simply take you to wiki pages that explain concepts by enlisting a wall of words. Instead, I attempted to synthesize different sources that really stretch outside the realm of basic physics and diversify the learning. Partially to aid myself, having a brain that sometimes operates quite slowly and on an elementary level, and also in honor of Feynman himself, most of these sources are far from “academic” or “scholarly”, and might be thought of as childish. I think these types of resources can often be the most illuminating. If you can’t distill something to simpler terms, I would argue you don’t understand it well at all, so why not start learning from the very fundamentals.
Intro
Popular misconception that science is an impersonal, dispassionate, and thoroughly objective enterprise
The scientific experience and its vast processes are diminished to a result, results are all that count, and the people that produce them are also too often overlooked
Science is people-driven (arguably more so than anything else?)
Are there not scientific trends and problems that characterize a specific age, just as something like fashion?
QED can mean quod erat demonstratum and be placed at the end of a mathematical proof, but it can also mean quantum electrodynamics
Quantum theory began with Max Planck in 1900 when the German physicist proposed that light and other electromagnetic radiation behaved like tiny packets of energy known as “quanta”. Prior, light had been regarded as waves
These quanta became photons
By the early 1930s quantum mechanics had created a mathematical model to describe the emission and absorption of photos by electrically charged particles such as electrons
Feynman turned his attention to perfecting the QED Theory; it still suffered from inconsistencies and calculations were known to give strange or infinite answers to reasonable physical questions
Making QED a sound theory required
Consistency with principles of quantum mechanics
Consistency with principles of the theory of relativity
Feynman took a unique and intuitive approach by creating his very own system of diagrams
Feynman diagrams are symbolic but powerful way of picturing the interactions between electrons, photons, and other particles
Most theoretical physicists rely on careful mathematical calculation to provide a guide into unfamiliar territory. With this in mind, Feynman comes off as cavalier and struts about seemingly reading nature like a book
Feynman displayed a “healthy contempt for rigorous formalisms”
Theoretical physics = one of the toughest intellectual exercises… combining abstract concepts that sometimes can’t even be visualized with extreme mathematical complexity?? No thank you
Apparently, Feynman was a lifelong prankster who treated authority and academic establishment with the same sort of disrespect he showed for mathematical formalism
Particularly Feynman hated “Why” questions
Fun side fascination with the quirky and obscure? Feynman had an obsession with the long-lost country of Tuva in Central Asia which he made a documentary on near his death (he also played, bongo drums, painted, frequented strip clubs, and deciphered Mayan texts)
When he died of cancer in 1988 his students at Caltech made him a banner: “We love you Dick.”
Upon being convinced to teach a series of freshmen lectures on physics, they were preserved in book form as The Feynman Lectures on Physics and proved a smashing success around the world
Perfect for the less mathematically or physically minded, Feynman is known for his ability to find just the right analogy or everyday illustration to extract the essence of a deep principle
Physics is rooted in the notion of laws = the existence of an ordered universe that can be understood by the application of rational reasoning
Laws of physics are not immediately transparent to us in our direct observation of nature
Feynman holds the considerable distinction of being one of the few to discover a new law of physics, one that pertains to the way that a weak nuclear force affects the behavior of certain subatomic particles
High-energy particle physics was the jewel in the crown of post-war science
This physics was glamorous and awesome with huge accelerator machines (check out CERN) and seemingly unending list of newly discovered subatomic particles
What unifies particle physicists? The role of symmetry and conservation laws
Symmetry of time: nothing in physics to distinguish one moment of time from the next. The world is “invariant under time translations” because regardless of your starting point (t=0 moment) there is no difference in the description of physical phenomena
The symmetry of time directly implies the most basic laws of physics: The Law of Conservation of Energy = you can move energy around and change its form but it is never created or destroyed
Quantum physics has dominated the 20th century
The above is indispensable in understanding subatomic particles, atoms, nuclei, molecules, and chemical bonding, the structure of solids, superconductors and superfluids, the electrical and thermal conductivity of metals and semiconductors, the structure of stars, shall I continue???
“Anybody who is not shocked by the [quantum] theory hasn’t understood it” – Niels Bohr, one of its founders
To mess with your brain: an electron cannot have a position in space and a well-defined speed at the same moment
CAN look for the location of an electron and find it
CAN measure its speed and obtain an answer
CANNOT make both observations at once
This indeterminism in the nature of atomic particles is encapsulated by Heisenberg’s uncertainty principle
The famous “two-slit” experiment teases out the wave-particle duality
Much easier to imagine an electron traveling directly from A to B, however, in reality it can take an infinite variety of routes and without observing which path is taken, we have to suppose that all possibilities contribute to the arrival point
Feynman’s “path-integral” or “sum-over-histories” approach to quantum mechanics
“There are 10^11 stars in the galaxy. That used to be a huge number. But that is only a hundred billion… we used to call them astronomical numbers. Now we should call them economical numbers.” – Feynman
Feynman scribbled a note to himself in Brazil in 1952: “first figure out why you want the students to learn the subject and what you want them to know, and the method will result more or less by common sense.” – I don’t know about you, but that makes me want to ask my professors and teachers a singular question, not meant with any hostility, but simply curiosity: “why do you want me to know this?”
IF you can’t reduce an idea to something more simple THEN you don’t really understand it
Feynman’s Preface
“Special problem” of his lectures was to maintain interest of the enthusiastic and smarter high school students entering Caltech
Just a side note: stultifying = to cause the loss of enthusiasm and initiative, especially as a result of a tedious or restrictive routine (AKA most high school physics classes)
Assumed knowledge of his lectures: geometrical optics and simple chemistry
All lectures were given without ever receiving any feedback from the students
Solutions to the diffusion equation
In more complex applications of quantum mechanics, such as electrical engineering and chemistry, the full machinery of the differential equation approach is not actually used
Energy bands = ranges of electron energy in a solid that that are so dense they appear to be continuous
“The power of instruction is seldom of much efficiency except in those happy dispositions where it is almost superfluous.” *superfluous = unnecessary, or being more than enough
The best teaching can be done only when there is a direct individual relationship between a student and a good teacher
1: Atoms in Motion
Physicists must accept that despite the 200 year accumulation of knowledge and laws, we do not yet know all of them, and there is an expanding frontier of ignorance surrounding physics
The correct statement of the laws of physics involves some very unfamiliar ideas which require advanced mathematics for their description; this requires immense amounts of preparatory training to learn what some laws and words even mean.
With an echo of Socrates, we know that we do not know all the laws of physics, so everything we know is only some sort of approximation
The principle of science = the test of all knowledge is experiment
Believe it or not, imagination is also needed to create accurate generalizations from the hints experiments allow us
Division of labor in physics
Theoretical physicists: imagine, deduce, guess at new laws but do not experiment
Experimental physicists: experiment, imagine, deduce, and guess
The “law” that mass is constant is actually inaccurate! Mass is found to increase with velocity, but appreciable increases require velocities near the speed of light. So yes, for ordinary speeds we can approximate mass to be constant, but it is not always true
WHAT is our overall picture of the world? Matter is made of atoms
The atomic hypothesis or atomic fact = all things are made of atoms – little particles that move around in perpetual motion, attracting each other when they are at a distance but repelling each other upon being squeezed together
Atoms in a water molecule are 2x10^-8cm in radius (this is an angstrom)
The “jiggling motion” of particles in an object is what we represent as heat and as heat increases, the jiggling increases
As demonstrated by classic textbook diagrams of a chamber with piston: when we compress a gas slowly, the temperature of the gas increases
In a solid material such as ice, there is a definite place for every atom. This special atomic arrangement in a solid is called a crystalline array
Most simple substances – with the exception of water and type metal – EXPAND upon melting because closely packed atoms separate, but an opened structure collapses in the case of water
As we decrease temperature, atoms approach a minimum amount of vibration at absolute zero but NOT this ≠ zero
Helium is special, it decreases its atomic motion as much as it can but at absolute zero there is still enough motion to keep it from freezing (unless the pressure is increased to make it solidify)
What happens at the surface of water?
There is always water vapor to be above liquid water
Air consists almost entirely of nitrogen, oxygen, some water vapor, and lesser amounts of carbon dioxide, argon, and other things
Molecules just above the surface of water interact with the liquid water and certain amounts are knocked with enough energy to evaporate
At the same time, some molecules from the vapor in the air stick to the water molecules in the liquid state and condense
Water molecules evaporate because they have more energy than the average, so the ones left have LESS average motion than before. In this way, the liquid also gradually cools if it evaporates
This process is complicated by stray oxygen or nitrogen molecules which work their way into the process of evaporation. When this occurs, the “air” dissolves into the water and if suddenly all of the air is taken away, they will leave more rapidly than they entered and will make bubbles in the process. This is what is so dangerous for divers!
What happens when you dissolve a solid in water?
Take salt (sodium chloride, NaCl) made of ions
An anion has a negative charge, meaning it has a few extra electrons
A cation has a positive charge, meaning it has lost a few electrons
Water is a polar molecule, meaning different parts have a partially positive or partially negative charge
Na+ and Cl- are then attracted to O2- and H+ ions in water respectively
This results in another dynamic process that is undetectable in diagrams: salt is dissolving in water as it also crystallizes out
More on the chemistry of solution and precipitation – can be complicated to predict which way a reaction will go, depends on the forward and backward reaction rate, and is also subject to the temperature and pressure at the point of the reaction
What about when atoms and ions “change partners?”
Atomic rearrangement = a chemical reaction (DISTINCT FROM A PHYSICAL REACTION)
Especially in reactions where large amounts of energy – kinetic energy – is generated, heat can produced from the combination of two atoms and result in burning
Heat is usually in the form of the molecular motion of the “hot” gas, but sometimes its magnitude generates light which is where our flames come from
Chemists can conclude that every substance is some unique arrangement of atoms
An interesting thought: we smell because a special molecule jostles along into our nose. Chemists can take these molecules (like the odor of a flower) and analyze them to determine the exact arrangement of atoms in space
We cannot picture all that is known about chemical arrangements, because atoms combine in three dimensions while we can only diagram two
Organic chemistry is the finest detective work ever done
Brownian motion = the perpetual jiggling of atomic particles
A key hypothesis: everything is made of atoms
So what follows: everything that animals do, atoms do.
This means: there is nothing that living things do that cannot be understood from the point of view that they are made of atoms acting according to the laws of physics
2: Basic Physics
Take a moment to appreciate everything occurring around you, as you enjoy (or not) these notes. As the observer you comprehend the words on this screen, this computer is extremely recent, an impressive addition, in the history of everything we know. Wherever you are there is air that you are breathing, of varying levels of moistness depending on where you are in the world. Even these few lines of writing, I have encapsulated more science and physics especially than you could begin to fully understand in a lifetime
A question Feynman asks: What common features do different movements have?
If we begin to gradually analyze things, we can hope to put together what at first appears different and thus reduce the number of different things thereby understanding them better
Scientific method = observation, reason, and experiment
What does it mean to understand something?
If “the world” (or at least what we believe to constitute it) is a chess game being played by the gods and we are only ever able to watch, maybe catching on to some of the rules along the way, than these rules of the game = fundamental physics
At first nature’s phenomena were roughly divided into classes ie heat, electricity, mechanics, magnetism, properties of substances, chemical reactions, light or optics, x-rays, nuclear physics, gravitation, meson phenomena, etc
Now the aim is to see complete nature as one set of phenomena
The basic problem in theoretical physics: find the laws behind the experiment and amalgamate these classes
Constant process of amalgamating a bit and then something new pops up
Example: heat and mechanics. Atoms that have more motion mean their system contains more heat, so heat and temperature effects can be represented by the laws of mechanics
Amalgamation = the act of unifying into one organization or structure
The simplest question becomes what are things made of and how few elements are there?
BEFORE 1920:
In the short-range, gravity is entirely too weak to impact particle level forces
Electrical force attracts and repels
All matter is a myriad of particles
Pressure comes from the collision of atoms
Random internal motions are heat
Waves of excess density are sound
There are 92* different kinds of atoms
*at the time of his lecture, now there are 118 unique atoms on the periodic table (pure elements), however, only 109 are stable.
Atoms contain a positive nucleus at the center which is surrounded by a certain number of electrons which are very light and negatively charged
The nucleus is composed of two kinds of particles: protons and neutrons (nearly the same weight and very heavy)
Protons are electrically charged while neutrons are neutral
Chemical properties depend on how many electrons are on the outside of the atom
Number of electrons = number of element = number on periodic table
The symbolic nomenclature of elements on the periodic table exists for human convenience
Complicating the description of electrical forces: the existence of a positive charge creates a condition in space so that when a negative charge is introduced, it feels a force. This is the electric field
The rules of an electric field:
Charges make a field
Charges in a field have forces on them and move
Electrical fields are complicated by magnetism (having to do with charges in relative motion)
Concepts of local and direct interaction
The concept of the electromagnetic field : carries waves, some waves are light, some are used in radio broadcasts, the general name for all = electromagnetic waves
Oscillatory in nature with various frequencies
Waves are distinctive due to their frequency of oscillation
The usual “pickup” that we get from electric currents in the circuits in the walls of a building has a frequency of ~ 100 cycles per second
AFTER 1920/QUANTUM PHYSICS:
At higher frequencies these waves behave more like particles (this behavior is explained by quantum mechanics)
Einstein created a the combination space-time to represent gravitation. It is curved
He changed the rules of particle motion: Newton’s laws of classical mechanics are wrong!
DISCLAIMER: things on a small scale act nothing like things on a large scale
Physics is difficult because human’s have no experience with the way things behave on a small scale – it is unnatural to us and we can only describe it analytically. Ironically, this takes lots of imagination
A particle cannot have a definite location and a definite speed in quantum mechanics
The uncertainty of momentum and the uncertainty of the position are complementary (the product of the two is bounded by a small constant, and written with a law like this: 𝛥x𝛥p ≥ h/2)
Why are atoms so big? How is the nucleus so small in comparison, yet accounts for nearly all the weight of the atom?
Using the uncertainty principle: IF all of the electrons were in the nucleus, we would know their position, but they must have a very large momentum, or kinetic energy which allows them to break away from the nucleus
Quantum mechanics also incited interesting changes in the the ideas and philosophy of science: it is impossible to predict exactly what will happen in any circumstance
Nature’s beauty and horror derives from the fact that it is fundamentally impossible to predict exactly what will happen in any given experiment despite following requisite conditions
Quantum mechanics unifies the idea of the field and its waves, and the particles
When the frequency is low, the field aspect is more evident and a more useful approximate description
When the frequency increases, the particle aspects become more evident
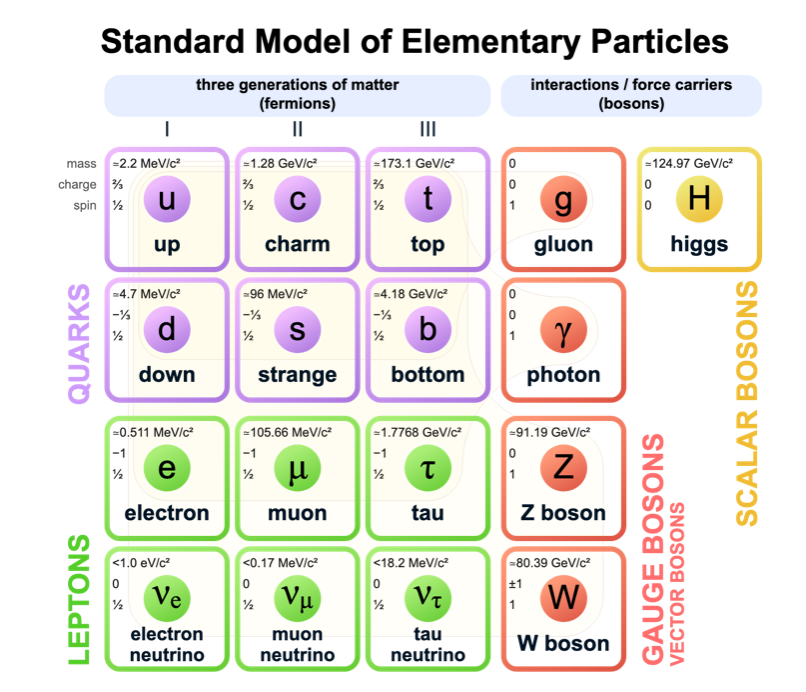
This gives us a new view of electromagnetic interaction and thus a new particle in addition to the electron, proton, and neutron! The photon!
Quantum-mechanically correct relationship between the electrons & photos is called quantum electrodynamics
QED = the fundamental theory of the interaction of light and matter or electrical fields and charges AKA the greatest success so far in physics. The following are all examples of this new law in action:
Collision of billiard balls
Motions of wires in magnetic fields
Specific heat of carbon monoxide
The color of neon signs
The density of salt
Reaction of hydrogen and oxygen to create water
Consequence of quantum mechanics as it relates to fundamental physics: waves behave like particles and particles behave like waves, there is no distinction between a particle and a wave
QED = the theory of all chemistry and of life (if life is reduced to chemistry and therefore to physics)
QED also predicts a lot of new things:
positron, or a particle with the same mass as an electron but of opposite charge. When the two come together they could annihilate each other with the emission of light or gamma rays
For each particle there is an antiparticle
Two numbers are put in and most of the other numbers in the world are supposed to come out (two numbers are the mass of the electron and the charge of the electron) BUT what about the atomic number? AKA how heavy the nuclei are, which is a number on the periodic table
Nuclei and Particles:
Enormous forces hold the nuclei together
When released the energy is HUGE compared to the chemical energy (think of an atomic bomb compared to a TNT explosion: the atomic bomb has to do with changes in the nucleus while TNT explosion has to do with the changes of electrons on the outside of the nucleus)
What forces hold the protons and neutrons together in the nucleus?
A field of sorts, when it “jiggles” it behaves like a particle
Suggests that other particles exist: cosmic rays, muon, pion,
Theory of quantum nucleodynamics is so difficult and complex that no one can work it out and it is left incomplete
There are about thirty particles that exist to our knowledge
Our inability to connect and understand their relationships with each other represents the amount of unconnected information we have without a good theory
QED = lots of knowledge but lots of uncertainty
Filling out the Mendeléev chart, and seeking a similar one for new particles
Chart of new particles made by Gell-Mann in the USA and in Nishijima, Japan. Basis of their classification is a new number, similar to the electrical charge, that is assigned to each particle called its “strangeness”, S
Historical choice: One Mev = 1.782 x 10⁻²⁷ gram
Elementary particles
What is zero mass? A particle with zero mass cannot be at rest
Although there are several different particles, there are four kinds of interactions between them:
Nuclear force (strongest) = between two or more nucleons, for example this force binds together the protons and the neutrons in atomic nuclei
Electrical interactions
Gravity (weakest)
To summarize: outside the nucleus our knowledge of physics seems quite expansive. Inside the nucleus, the principles of quantum mechanics have not been found to fail, but there is much much more we do not know
We base all of our knowledge on the premise of relativistic space-time
We do not know how the universe got started
There has never been an experiment made to check our ideas of space and time accurately, below a certain tiny distance, so we only really “know” our ideas work above that distance.
The rules of our aforementioned chess game are the quantum-mechanical principles which apply – so far as we know – to new particles and old particles
Studying the origin of the forces in the nuclei lead us to new particles, but they appear in great profusion (large quantity or abundance) and we don’t have a complete understanding of their interrelationships
We are progressing towards an understanding of subatomic particles, but slowly, and with no conception of how far there is to go
3: The Relation of Physics to Other Sciences
Most fundamental and all-inclusive of the sciences
Present-day equivalent of what used to be called natural philosophy
Physics has remarkable relationships with engineering, industry, society, and war
Very special relationship with mathematics… not a science to us, in the sense that experiment doesn’t prove its validity, or it is not a natural science
Feynman wants it to be clear: just because something is not a science doesn’t mean that it is bad! His example, love is not a science
CHEMISTRY
Arguably the most deeply affected by physics
In the early days, chemistry was almost completely devoted to inorganic chemistry (not associated with living things)
Because the interrelationships of elements and the rules by which substances are combined are ultimately explained by quantum mechanics, in principle, theoretical chemistry is basically physics
There is a branch of physics and chemistry developed by both sciences together: statistical mechanics
Statistical mechanics boils down to the science of heat or thermodynamics
This reduces inorganic chemistry to physical chemistry (to study the rates at which reactions occur and what is happening in detail) & quantum chemistry (to help us understand what happens in terms of the physical laws)
Then there is organic chemistry which studies all of the substances associated with living things
These are the same atoms, just in more complicated arrangements
“Orgo” as it is commonly referred to has a very close relationship to biology
Principles are shared with inorganic chemistry, but the studies and focus of organic chemistry take it more into the direction of biochemistry and molecular biology – with a focus on the analysis and synthesis or the substances formed in biological systems (living things)
BIOLOGY
As we have heard 1000 times over in our lives: “Biology! The study of living things!”
Early days saw biology has a purely descriptive problem of finding out what things were with rudimentary means such as counting limbs and observing the physicality of different organisms
Next level was a focus on the machinery inside living bodies
Relationship between physics and biology gets interesting here, as the latter aided the former in discovering conservation of energy (this was first demonstrated by Mayer in connection with the amount of heat taken in and given out by a living creature)
Off the top of your head, it is quite easier than you might think to pinpoint physical phenomena in the biology of living animals: circulation of blood, bodily pumps, pressure, nerves, etc
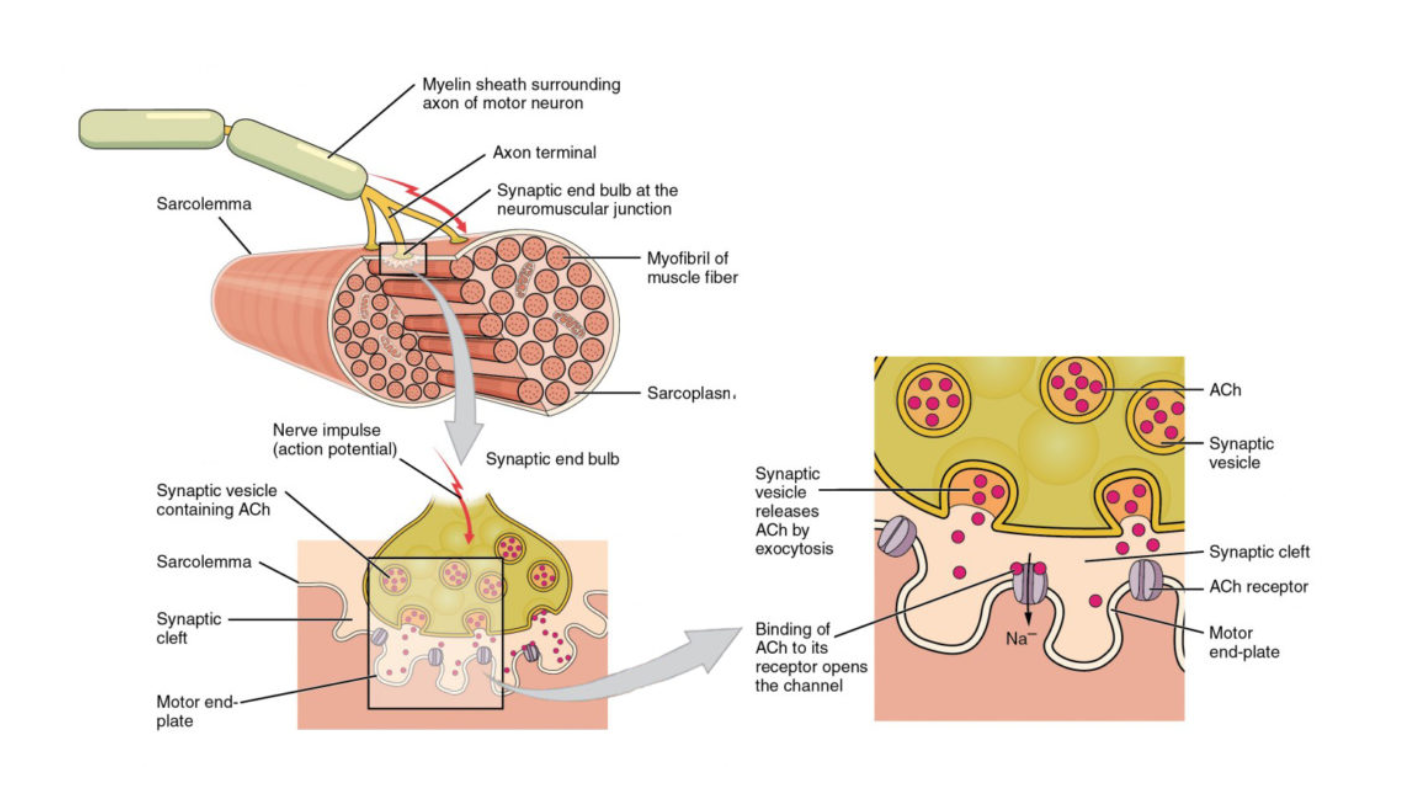
Nerves = fine tubes with complex yet very thin walls. Cells pump ions through these walls so that there are positive ions on the outside and negative ions on the inside (similar to a capacitor)
If this membrane “discharges” in one place, so that the electric voltage is reduced there, it has a cascading electrical influence on its neighbors and creates a wave of “penetrability” of the membrane that runs down the fiber whenever it is “excited” at a particular place
These are how we transmit messages through the body
In the nerve cell there are processes that pump the ions slowly out again to get the nerve ready for the next impulse
Anytime electricity is involved, physics is present, and therefore it has a great deal of influence on understanding this signaling
Messages that originate in the brain are sent out along nerves
Nerves branch out into fine little things that are connected to an endplate structure near a muscle
When the messages reach these areas a chemical release of acetylcholine is triggered to make muscle fibers contract
The fundamental processes in the muscle that make mechanical motions are not known because we don’t fully understand the machinery by which the chemical reaction induced by acetylcholine can actually modify the dimensions of the muscle
Biology is an enormously wide field (if you didn’t already assume such)
What does light do in the eye?
How does hearing work?
A really complex one: how does thinking work??
To give some perspective, even the biology humans study that is considered pretty fundamental, such as the way nerves work, is not actually so crucial. Living things can exist without nerves… take plants!
The most common feature of all living things is that they are made of cells
Each cell contains complex machinery for doing things chemically
It is often hard to recreate certain reactions in laboratory settings because of high energy barriers
Feynman describes enzymes as very large molecules within cells which in some complicated way hold the smaller molecules just right so that reactions can occur easily
Enzymes used to be called ferments because they were discovered in the fermentation of sugar
Enzymes are large, unique, reusable, and made of protein
Not all proteins are enzymes but all enzymes are proteins
They are not directly related to reactions and act solely as the catalyst for one atom to move places (given adequate supply and a proper place to be moved)
Just a reminder of why physics is so important in biology and other sciences – the experimental techniques that were developed and all of the classification and labeling of atoms that resulted
Point in case: isotopes (having different atomic weights based on differing numbers of neutrons in the nucleus)
Proteins = a chain, or series, of amino acids
There are twenty different amino acids, all can combine with each other to form chains with a CO-NH backbone
One of the greatest triumphs of Feynman’s time was the discovery of the exact spatial atomic arrangement of certain proteins
How does an enzyme know what to be? The transmission of instructions is done by a substance in the nucleus of the cell called DNA
Sperm cells consist mostly of DNA
If DNA is thought of as a blueprint – one of the most common analogies used to first teach the concept to anyone – it must be able to do two things: first, reproduce itself and second, instruct the protein
DNA was studied chemically to determine its composition and with the use of x-rays to reveal its pattern in space
The DNA molecule is a pair of chains twisted upon each other
The backbone of each chain is a series of sugar and phosphate groups
Specific series of the DNA = specific genetic instructions it carries and transmits
For the sake of the chain analogy, DNA has specific links that attach in specific ways to link the two chains: adenone, thymine, cytosine, and guanine
During reproduction, when a chain is split and each goes along with its new cell, the remaining half-chain creates a complementary new chain
The central unsolved problem of biology in Feynman’s day was how do the specific arrangements and connections of bases determine our genetic make-up?
Ribosomes are the tiny particles in the cell in charge of protein synthesis, however, they are not in the nucleus with the DNA
Tiny little molecule pieces also come off of DNA, they are not as long or large as the DNA itself, more of a mere section, and known as RNA
The RNA carries a message as to what kind of protein to make and travels with this to the ribosome
ASTRONOMY
Older than physics
Most remarkable discovery was that the stars are made of atoms of the same kind as those on earth
Using a spectroscope we can analyze the frequencies of the light waves and differentiate atoms in the stars
Two chemical elements were discovered on a star before they were discovered on earth: helium (the sun) and technetium (on certain cool stars)
Because we know about the behavior of atoms under conditions of high temperature and low density, we can analyze via statistical mechanics the behavior of stellar substances
Obviously conditions cannot be reproduced on earth, but physics enables us to make very precise estimates of what will actually happen in different circumstances
Ironically, we understand the distribution of matter in the interior of the sun far better than the interior of the earth
What is the origin of the energy of stars? What makes them able to continue to burn?
Nuclear reactions are occurring in the stars (some man was out walking with his girlfriend when he discovered this)
The nuclear burning of hydrogen supplies the energy of the sun (hydrogen is converted to helium)
The manufacture of carious chemical elements proceeds in the centers of stars, from hydrogen
The elements that created our earth were once “cooked” inside a star and spit out – we know this because of a very important clue: the proportion of different isotopes is never changed by chemical reactions, they are a result of nuclear reactions
Stellar explosions like the one that made earth are called novae and supernovae
GEOLOGY
Known as an earth science
Examples include meteorology and the weather, these sciences have physical instruments (thanks to the development of experimental physics)
The concept of predicting the condition of our air is scrutinized by physicists (technically we don’t even know exactly what is happening with our air as we speak because it is constantly moving and swirling around us)
The situation of turbulent flow arrises in many fields and remains unanalyzable
Basic geological question: what makes the earth the way it is?
An obvious processes is the erosion due to water and air
Processes that counter erosion also occur… otherwise all of our mountains would be gone by now!
Theory that there are currents inside the earth which are circulating due to the difference in temperature inside and outside
If these currents are circulating in opposite directions, matter will collect in the region where they meet and make mountain belts that if unhappily stressed, will produce volcanoes and earthquakes
We know the speed of earthquake waves through the earth and the density distribution of the earth, BUT physicists have been unable to theorize exactly how dense a substance should be at the pressures expected near the center of the earth
AKA we don’t know the properties of matter existing in these circumstances
Feynman seems to think it is only a question of when someone is mathematically gifted enough to work out exactly how matter behaves inside our earth – not if just when
PSYCHOLOGY
“Psycho-analysis is not a science: it is at best a medical process, and perhaps even more like witch-doctoring”
Has a theory for what causes disease
Psychoanalysis has not been carefully checked by experiment, so there is no certainty of the number of cases where it works and where it doesn’t work
Other branches include the physiology of sensation (what happens in the eye, brain, etc)
The central problem of the mind (or nervous system): when an animal learns something, it can do something differently than before and its brain cells must have changed. As these cells are made of atoms, in what way is it different?
When a fact is learned, a memory stored, or a feeling had, we don’t know where to look or what to look for physically
Analog of the brain and computing machines: elements with a lot of lines similar to the synapse (connection of one nerve to another)
Feynman seems to be overwhelmed by the mental science of humans, because he concludes that all human beings are “so different” and it will be a long time before we are able to understand the complexity of human behavior. For good measure he adds that dogs are easier to understand, but nobody knows how dogs work yet either.
In my opinion, Feynman doesn’t seem to do this field of science justice. He seems to diminish it to the psychoanalysis of Freud, and doesn’t consider any of the tangible studies done related to the human brain, trauma, addiction, stress, etc
Physics is clearly important to other sciences simply in the contribution and invention of instruments
In order for physical theory to be of any use we must know were the atoms are located
There is a kind of problem in other sciences that does not exist in physics – a historical question. How did it get that way?
The theory of evolution of the question of the origins of the universe are problems that don’t exactly exist within physics. The laws of physics aren’t questioned – right now.
A fundamental problem of physics still being solved: finding new particles
The analysis of circulating or turbulent fluids
We can predict a lot of things related to fluid dynamics, but when water is really running through a pipe, we can’t quite explain it
Feynman believes poets do not write to be understood
“The whole universe is in a glass of wine” – the twisting liquid evaporates depending on the wind and weather, the reflections in the glass, the atomic composition and how it got its particular chemical elements to be the way they are through fermentation, a product of the substrates with the help of enzymes
All life is fermentation
Our minds like the convenience of dividing life into parts that are more manageable for us, like physics vs biology and chemistry and geology, astronomy, and psychology. However, nature doesn’t know this, or behave in accordance. Let’s put everything back together!
4: Conservation of Energy
What is energy?
The conservation of energy governs all natural phenomena that are known to date – with no exception in so far as we know
Law states that there is a certain quantity called energy that does NOT change along with the manifold changes in nature
The idea is abstract because it is a mathematical principle
The energy can be thought of as a numerical quantity which does not change when something happens
When calculating the energy of a system, sometimes it leaves and sometimes it enters
Energy has a large number of different forms (eg gravitational, kinetic, heat, elastic, electrical, chemical, radiant, nuclear, mass)
In physics today we still have no knowledge of what energy is
The complex formulas we have for calculating energy always give us a constant numerical representation for it, but energy is an abstract thing because it does not tell us the mechanisms or the reasons for the various formulas
GRAVITATIONAL POTENTIAL ENERGY
Exists near the surface of the earth
GPE = weight x height because it represents the energy which an object has because of its relationship in space, relative to the earth (this formula holds true so long as we are not too far from the earth as the force weakens as we go higher)
The reasoning for this form of energy is beautiful, but it assumes that perpetual motion is not possible. Experimentally it has been checked to be true.
The general name of energy which has to do with its location relative to something else is called potential energy
If it is a question of any other kind of force, take electrical, just tack on “potential” to the end
The general principle for change in energy is: Δenergy = force x (distance force acts through)
The conservation of energy can be used to deduce what happens in a number of circumstances, including the class high school problems and laws about pulleys and levers
Stevinus also discovered a clever way to solve these kinds of problems
KINETIC ENERGY
Consider the classic pendulum in its motion, it loses height swinging from side to side. Where does this potential energy go?
The motion at the bottom of the pendulum must be a quantity of energy that permits it to rise to a certain height, and has nothing to do with the machinery or the path by which it comes up to that height
KE = weight x (velocity)² / 2 x gravity (because of the implication of gravity in the formula, you must consider the relativistic correction at high speeds
The existence of energy can be illustrated in many forms
Elastic energy – work is required to pull a spring down, and once it is down, it can be used to lift weights (in its stretched condition is has a possibility of doing some work)
Elastic energy equations are complicated by the tension the spring is experiencing, and that is why the simple (weight x height) formulas are usually incorrect
When you let go of a spring, it passes through an equilibrium point (in mathematics this is the constant solution to a set of differential equations) and is converted to kinetic energy where it goes back and forth between compressing or stretching the string and kinetic energy of motion
Eventually the motion stops! Why? Where does the energy go?
The kinetic energy of atoms is constantly happening in the background, we just lose track of it because it is not visible
So how to be certain that the kinetic energy is still there? Use a thermometer
If something is warmer –> there is an increase of kinetic energy by a definite amount
In this way, heat energy is just kinetic energy on an invisible scale, or internal motion
An issue with our experiments with matter on a large scale: we cannot fully demonstrate the conservation of energy because every time we move a “large clump of stuff” the atoms do not remain absolutely undisturbed and a certain amount of random motion goes into the atomic system. This cannot be seen. But it can be measured using change in temperature!
Many many forms of energy
Electrical energy has to do with pushing and pulling electric charges
Radiant energy is the energy of light, and we know it as a form of electrical energy because the light can be represented as wiggles in the electromagnetic field
The energy that is released in chemical reactions is known as chemical energy
Chemical energy is understood to have two parts: kinetic energy of the electrons inside the atoms, and the electrical energy of the interaction between electrons and protons inside the atoms
Nuclear energy is involved with the arrangement of particles inside the nucleus (for which we have formulas but no fundamental laws)
Associated with the relativity theory is a modification of the laws of kinetic energy – it is combined with another thing called mass energy
Basically, anything has energy from its sheer existence!
This all might be more familiar with Einstein’s equation: E = mc²
You might be wondering, like me, if there are any other conservation laws in physics…
We don’t understand any of these laws deeply; we do not understand energy as a certain number of little blobs
The energy of a photon is Planck’s constant x frequency
As we presently understand, there can be any amount of energy
In quantum mechanics it gets even more interesting because it turns out the conservation of energy is very closely related to another important property of the world, things do not depend on the absolute time
This basically means that you can set up an experiment one moment and then later on do the exact same experiment and have it behave the exact same way, regardless of the different start times. We don’t know if this is strictly true though
The conservation of charge (merely counting how many positive – how many negative electrical charges you have, and the number is never changed)
The conservation of baryons – baryons encompass a number of strange particles like the neutron and proton. In any reaction, it appears that counting the number of baryons coming into a process will result in the same number coming out *if you count anti baryons as -1 baryon
The conservation of leptons (electrons, mu mesons, and neutrinos, and positrons, as anti-electrons, are -1 leptons)
Of the six conservation laws, three subtly involving space and time and three are simple in the sense that they count something
With regard to conservation of energy, note that available energy is another matter (we know energy is conserved, but the energy available for human utility is not so easily conserved)
The laws which govern how much energy is available are called Laws of Thermodynamics and involve the concept of entropy for irreversible thermodynamic processes
So where can we get our supplies of energy today? From the sun, rain, coal, uranium, and hydrogen – but mostly from the sun because it makes the rain and the coal.
Nature liberates a lot of energy from the sun, but only one part in two billion falls on the earth
The energy from hydrogen comes at explosive and dangerous conditions and can only be controlled in thermonuclear reactions
Get this: the energy that can be obtained from 10 quarts of water per second is equal to all of the electrical power generated in the United States *at least it was in Feynman’s time 5: The Theory of Gravitation
Apparently this chapter will discuss some of the most far-reaching generalizations of the human mind
Is it not quite elegant and impressive that nature follows with such completeness the simple principle of the law of gravitation?
Every object in the universe attracts every other object with a force that (for any two bodies) is proportional to the mass of each and varies inversely with the square of the distance between them
Mathematically: F = G x mm’/r²
An object responds to a force by accelerating in the direction of the force by an amount that is inversely proportional to the mass of the object
The story of this law and its two important principles begins with the ancients observing the motions of the planets among the stars, and finally deducing that they went around the sun (a fact later discovered by Copernicus)
Tycho Brahe had a different idea, he proposed that the debates about the motions of the planets would best be resolved if the actual positions of the planets in the sky were measured sufficiently accurately
Kepler was a mathematician that succeeded Brahe and from his data, discovered more simple and beautiful laws regarding planetary motion
Kepler’s laws:
Each planet goes around in the sun in a curve called an ellipse with the sun at a focus
Ellipses are not just ovals, but very specific and precise curves that can be obtained using two tracks, one at each focus, a loop of string, and a pencil. Mathematically: it is the locus of all points the sum of whose distances from the two fixed foci points is a constant
He also observed that the planets do not go around the sun at a uniform speed, but move faster when they are nearer the sun and more slowly when they are farther from the sun
Radius vector = a line drawn from the sun to any point in a planet’s orbit
If the planets went in circles, which they nearly do, the time required to go around the circle would be proportional to the 3/2 power of the diameter
Each planet moves around the sun in an ellipse, with the sun at one focus
The radius vector from the sun to the planet sweeps out equal areas in equal intervals of time
The squares of the periods of any two planets are proportional to the cubes of the semi major axes of their respective orbits: T ∝ a³∕²
While Kepler was discovering his laws, Galileo was studying the laws of motion
In these times, people theorized that planets orbited because invisible angels were behind them beating their wings
The principle of inertia aided the process of refining this theory – if something is moving, with nothing touching it and if left completely undisturbed it will go on forever, coasting at a uniform speed in a straight line
Newton modified the above with his forces that are able to change the motion of a body
If a body speeds up, a force must be applied in the direction of motion
If a motion is changed to a new direction, a force must have been applied sideways
Thus, the more massive something is, the stronger the force required to produce a given acceleration
The idea resulting from all of these considerations of forces is that no tangential force is needed to keep a planet in its orbit
Inertia means that the force needed to control the motion of a planet around the sun is not a force around the sun but a force towards the sun (if undisturbed the planet would go off in a straight line, so there must be a force acting perpendicularly towards the sun so the resulting path is this circular orbit)
NEWTON’S LAW OF GRAVITATION
Newton appreciated that the sun could be the organization of forces that govern the motion of the planets
His observations inspired the idea that all forces are directed exactly towards the sun
Using Kepler’s third law, it is possible to show that the farther away the planet, the weaker the forces
Newton was a man of “considerable feeling for generalities”
Knowing that there was a force holding us to the earth, he proposed that this was a universal force that everything pulls everything else
The radius of the moon’s orbit = ~240,000 miles
It takes ~ 29 days for the moon to go around the earth… a month?
This messes with my brain: the moon ‘falls’ in the sense that it falls away from the straight line it would pursue if there were no forces
Again: if you shoot a bullet faster and faster, because the earth’s surface is curved, when the bullet falls its standard 16ft due to gravity & forces, it may be at just the same height above the ground as it was before because it is traveling “around” the curved earth
A handy geometry theorem says that a tangent to a circle is the mean proportional between the two parts of the diameter cut by an equal chord
“Any discovery of a new law is useful only if we can take more out than we put in” – Feynman
The pull of the moon on earth causes the tides (they go up and down in 12 hours)
What is a centrifugal force?
Gravity is responsible for the earth being round (they are nearly spheres, but should technically be elliptical)
Jupiter’s moons and their inability to be on schedule almost caused the death of Newton’s laws of gravitation. If a law does not work in even one place then it is simply wrong.
Jupiter’s moons were ahead of schedule when it was close to the earth, and they were behind schedule when Jupiter was further from the earth. Why?
The time it takes for us to see the moons of Jupiter differs because of the time it takes light to travel from Jupiter to earth depending on their distance from each other
So, light does not travel instantaneously
This also allowed for the first estimate of the speed of light in 1656
Do stars experience gravitational attractions as planet’s do? Yes thanks to a double-star system
One of the most beautiful things in the sky is a globular star cluster
When you inspect an entire galaxy there is an obvious tendency for its matter to agglomerate (cluster together around a common center)
That does not mean it is just a ball of matter because everything is spinning and has angular momentum
Galaxies have a scale of perhaps 50,000 to 100,000 light-years
The earth’s distance from the sun is 8 1/3 light-minutes
Galaxies also cluster together most likely due to gravitational forces just as stars cluster within galaxies
The law of gravitation also gives some ideas on the origin of the stars: if a big cloud of gas and dust exists, the attraction of tiny pieces together causes clumps to form. These clumps are basically fetus stars
CAVENDISH’S EXPERIMENT
Performing simple experiments to observe forces of gravitation is actually quite difficult because of all the confounding variables you must address like keeping all air out, making sure nothing is electrically charged, etc
Cavendish designed an apparatus that did just that and first demonstrated the direct force between two large, fixed balls of lead and two smaller balls of lead on the ends of an arm supported by very fine torsion fiber
He measured how much the fiber twisted to indirectly measure the strength of the force and verify that is was indeed inversely proportional to the square of the distance
This allows one to accurately determine the coefficient G in the formula F = Gmm’/r²
His experiment allowed us to discover the mass of the earth by rearranging the equation!
GRAVITY
What is the machinery of gravity?
Even though Newton created these laws on gravity, he never explored what created the forces, only that they existed.
It is quite characteristic of many physical laws to have this abstract character: someone makes an amazing and beautiful discovery, does enough math and experimentation to ensure it can become a fundamental law, and continues along
No one knows why we are able to use mathematics to describe nature without a tangible mechanism behind it
Although gravitation and other forces are similar, there is no explanation of it in terms of other forces
The unified field theory is an elegant attempt to combine the forces of gravitation and electricity as they behave rather similarly (the forces are constant, and vary inversely as the square of the distance, they also behave in the opposite direction as ‘likes repel’)
Nature’s universal charge = the repulsion of two electrons due to electricity and the attraction of two electrons due to their masses
Can measure the ratio of electron repulsion: gravitational attraction
The above ratio is independent of the distance and is a fundamental constant of nature (1 / 4.17 x 10⁴²)
It has been proposed that the gravitational constant is related to the age of the universe, but the fact that standard measurements of time (such as years) were created by humans complicates the ability to make further calculations
Is it possible that the gravitational constant is changing with time? This would be a fairly logical deduction if it is indeed related to the aging of the universe… BUT that would mean that at the time of the earliest records of life on earth that we have, the earth would’ve been much closer to the sun, so close that temperatures would have been 100ºC hotter and therefore the aforementioned life wouldn’t have existed. So no, probably not related to age
For all substances tested thus far on record, masses and weights are exactly proportional within 1 part in 1,000,000,000 or less
GRAVITY AND RELATIVITY
Einstein modified Newton’s law of gravitation
This modification corrected it (yes Newton was wrong!) to take the theory of relativity into account
Anything which has energy has mass (mass in the sense that it is attracted gravitationally)
This includes light!
When a light beam, which has an energy, passes the sun, there is an attraction and thus it does not travel straight but is deflected
Quantum-mechanical aspects of nature also fail to explain gravitation
When the scale is so small that quantum effects become relevant, gravitational effects become irrelevant
Newton’s law was modified to Einstein’s law which really should be further modified with the uncertainty principle, however, this has yet to be done
______________________________________________________________________
6: Quantum Behavior
ATOMIC MECHANICS
The theory of the index of dense materials and total internal reflection
The “classical theory” of electric waves turns out to be a completely adequate description of nature for a large number of effects
Light energy comes in lumps called photons
Classical and older theories tend to fail to describe the behavior of larger pieces of matter because they are really made up of atomic-sized particles
Quantum mechanics is the description of the behavior of matter in all its details – in particular things happening on an atomic scale
These things on a very small scale do not behave like anything we have experience with: not particles, not clouds, not balls, weights on springs, etc
Newton thought light was made up of particles, then it was discovered that it behaves like a wave, later then again it was found to sometimes behave like a particle and so now we roughly conclude it isn’t exactly either
There is some generalization to be made: electrons behave just like light because the quantum behavior of atomic objects is all the same (electrons, protons, neutrons, photons ‘of light’, etc)
The accumulation of info about atomic and small-scale behavior was gradual, and the increasing confusion was resolved in 1926-27 by Schrodinger, Heisenberg, and Born when they obtained a consistent description for matter
Just like trying to picture things in four dimensions is nearly impossible for humans, atomic behavior is so unlike ordinary experience that it is very difficult to get used to
AN EXPERIMENT WITH BULLETS
Behavior of bullets = particle like, behavior of water waves = wave like
Set up a wall with two holes in it and backstops to determine where bullets that are sprayed out of a machine gun land, and collect them
Can then find the probability that a bullet which passes through the holes in the wall will arrive at the backstop at a certain distance, x, from the center
Must mention probability because there is no way to be definite about where each bullet will go
Take the ratio of (no of bullets which arrive at the detecter in a certain time: total no of bullets that hit the backstop during the same time)
The bullet experiment observations reveal that probability of arrival shows no interference (P₁₂ = P₁ + P₂)
AN EXPERIMENT WITH WAVES
A small object that creates waves jiggles around in a shallow trough of water to make circular waves
There is another wall with two holes in it and something to absorb the waves so there is no reflection
Need a detector of sorts that measures the energy being carried by the wave
The detector picks up the wave motion and calculates the proportional intensity at the rate in which waves approach it – regardless of the magnitude
As holes are covered or opened, a very different relationship is discovered between interference and intensity
When intensity has its maxima, the waves are in phase (peaks of the waves add together to give a large amplitude and subsequently, a large intensity)
If two waves arrive with a phase difference of pi they are out of phase and the resulting motion is the difference of the two amplitudes
For waves: I₁ = |h₁|² , I₂ = |h₂|² , I₁₂ = |h₁ + h₂|²
|h₁ + h₂|² = |h₁|² + |h₂|² + cosδ, where δ is the phase difference between h₁ and h₂
One last way to write is would be: I₁₂ = I₁ + I₂ + 2√ I₁ I₂cosδ which is the “interference term” – intensity of these water waves can have any value and it shows interference
AN EXPERIMENT WITH ELECTRONS
Same set up as before, where an electron gun of sorts is shot through two holes in a wall and the ones that make it through are intercepted by a detector of sorts
a geiger counter is recognizable by its erratic clicking sounds
The rate at which the “clicks” of the detector may change, but the size or loudness of each click is always the same
Electrons always arrive in identical lumps
What is the relative probability that an electron ‘lump’ will arrive at the backstop at various distances, x, from the center?
The probability distribution is the same as was found for waves!
Because each electron either goes through hole 1 or through hole 2, the observed curve to map their probability must be a sum of the electrons that come through both, and we can conclude that there is interference
The mathematics for describing the electron probability distribution curb is the exact same as for describing waves BUT what actually goes on, or how it happens remains an inexplicable mystery
The electrons arrive in discrete lumps, like particles, and the probability of their arrival is distributed like that distribution intensity of a wave – hence the particle-wave duality of electrons
In quantum mechanics, it turns out that the amplitudes HAVE to be represented by complex numbers because the real parts alone are not sufficient
Modify the experiment by adding a very strong light source because we know that electron charges scatter light
This means that when an electron passes we will be able to detect it because it will scatter light in a way that is visible to us
This modification to the experiment allows us to conclude that when we look at the electrons, the distribution is different than when we do not look
Why?! How? Well, the light source is strong enough to disturb the delicate electrons. The electric field of light acting on a charge will exert a force on it
It becomes a catch-22: if you turn down the brightness of the light source, the waves will be weaker and will not disturb the electrons as much, but we will not be able to observe the negligible effect anymore
However, using a dimmer light source does reveal some interesting things: the flash of light scatter from the electrons as they pass does NOT get weaker (it is always the same size). Sometimes electrons will pass through without a visible flash!
This really means that the light also acts like electrons… it arrives in a scattered manner of lumps as well, called photons
As the intensity of light is turned down, it doesn’t change the size of the photons, only the rate at which they are emitted
Further analyzing the experiment, if the electrons are not seen (ie they are not disturbed by a bright light source), interference occurs
Remember that the momentum carried by a “photon” of light is inversely proportional to its wavelength (p = h/λ)
If you want to disturb electrons only slightly try lowering the frequency of the light instead of its intensity
This would mean using light with a more red color, or infrared, or radio-waves/radar
Using these longer waves, we see a big fuzzy flash when light is scattered by electrons and can no longer tell which hole it went through because the wavelength itself was longer than the distance between holes
In conclusion, it is impossible to arrange light in such a way that we can tell which hole the electron went through and still not disturb the pattern
This led to Heisenberg’s suggestion that these laws of nature could only be consistent if there were some basic limitation on our experimental capabilities – the uncertainty principle explains that “it is impossible to design an apparatus to determine the path an electron takes that will not at the same time disturb the electrons enough to destroy the interference pattern”
The complete theory of quantum mechanics which we use to describe atoms and all matter depends on the correctness of this uncertainty principle
Returning to the bullets, we didn’t see any interference patterns there, so does that mean they don’t confine to these constructs for describing the behavior of nature? No.
The bullets just had wavelengths that were so tiny we didn’t detect the interference and the curve appeared smooth
A kind of average = the classical curve (of distribution by Maxwell-Boltzmann)
FIRST PRINCIPLES OF QUANTUM MECHANICS
A summary of findings based on an ideal experiment:
The probability of an event is given by the square of the absolute value of a complex number Φ which is called the probability amplitude
P = probability
Φ = probability amplitude
P = |Φ|²
When an event can occur in several alternative ways, the probability amplitude is the sum of the probability amplitudes for each separate event and there is interference
Φ = Φ₁ + Φ₂
P = |Φ₁ + Φ₂|²
If an experiment is performed which is capable of determining whether one or another alternative is actually taken, the probability of the event is the sum of the probabilities for each alternative and the interference is lost
P = P₁ + P₂
If you ask how it works exactly you will be disappointed because no one has discovered the machinery behind the law
A very important difference between classical and quantum mechanics: it is impossible to predict exactly what would happen, you can only predict the odds!!!
So has physics given up? This recognition that only the prediction of the probability of different events occurring, and not the prediction of the events themselves seems a retrenchment, but it is unavoidable
This puzzle is the way that nature is
THE UNCERTAINTY PRINCIPLE
Heisenberg originally stated: If you make the measurement on any object, and you can determine the x-component of its momentum with an uncertainty of 𝛥p you cannot, at the same time, know its x-position more accurately than 𝛥x ≥ h/2𝛥p
In other words, the uncertainties in the position and the momentum at any instant must have their product greater than half the reduced Planck constant
Basically, the uncertainty principle protects quantum mechanics by confirming and perpetuating our inability to accurately know where something is (position) and how fast it is traveling (momentum) at exactly the same time